Abstract
While it is increasingly becoming clear that cancer cells reside in symbiosis with diverse stromal and immune cells, the tumor supportive microenvironment (TSM) in acute myeloid leukemias (AML) remains poorly understood. We and others previously identified that the presence of tumor-supportive macrophages (often with an M2-like phenotype) was associated with poor prognosis and a more aggressive course of disease in vivo using various AML models. Therefore, tools to target the tumor supportive microenvironment, or to convert an M2-polarized TSM into a tumor suppressive one, are of great interest. Here, we investigated the effects of therapeutic drugs on AML blasts and their TSM specifically focusing on the macrophage compartment.
Drug screens using 20 candidate compounds including metabolic inhibitors (targeting glycolysis and mitochondrial respiration) were performed on human AML cell lines (n=16), primary AML samples (n=52), and CD34+ cells from healthy cord blood donors (n=5). Experiments were performed in co-culture using MS5 stromal cell as a supportive feeder. Effects on cell viability and mitochondrial membrane potential (MMP) were evaluated by flow cytometry on blasts (CD45+CD34+) as well as on M1 or M2 macrophages (CD80+ or CD163+CD206+ within the CD45+CD34- fraction). Effects on glycolysis and oxygen consumption were evaluated by Seahorse. Human primary monocytes isolated from healthy donors were also used for co-culture experiments and M1 and M2 macrophages were generated as previously described (Weinhäuser et al, 2022).
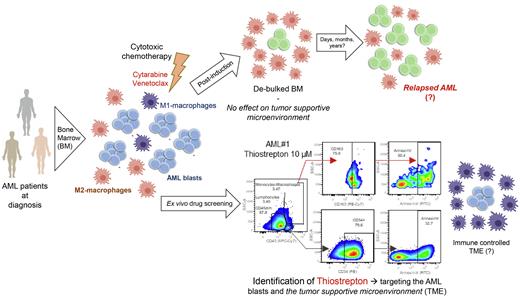
We identified that conventional drugs such as cytarabine and venetoclax had significant cytotoxic effects on AML blasts, albeit with heterogeneous efficacies dependent on AML subtype, while M2 macrophages remained largely unaffected. Functionally, M2 populations displayed higher MMP levels compared to AML blasts, presumably contributing to their increased drug resistance. Among all the evaluated compounds, only thiostrepton (an antibiotic that also inhibits FOXM1), KPT-9274, and metformin displayed cytotoxic effects on both the AML blasts and M2 macrophages, associated with reductions of MMP levels in both compartments.
Various studies have aimed to identify compounds that would repolarize tumor supportive M2 macrophages into tumor suppressive M1 macrophages, including thiostrepton. Using healthy M2 macrophages, we could not detect consistent signs of re-polarization (evaluated by flow cytometry and gene expression profile) upon thiostrepton treatment. In fact, none of the 20 tested compounds was able to efficiently repolarize M2 into M1 macrophages. Furthermore, when we evaluated the effects of thiostrepton pretreated M2 macrophages on short-term and long-term AML cultures, we could not detect a significant reduction in AML cell viability or proliferation as observed for M1 co-cultures. However, we did notice a pronounced cytotoxic effect on the M2 macrophages themselves when a higher dose of thiostrepton (>5 µM) was administered. Metabolically, thiostrepton-treated macrophages also displayed decreased mitochondrial respiration. Next, we determined the ED50 of a large panel of AML cell lines (n=16), which ranged from 1.91-38.67 µM suggesting that tumor cells themselves display considerable sensitivity to thiostrepton. Cell line specific ED50 values were correlated to their transcriptomes and gene set enrichment studies revealed that thiostrepton resistance was associated with the term "ACTIVATION OF GENE EXPRESSION BY SREBF and SREBP", suggesting that fatty acid metabolism can be targeted to enhance thiostrepton efficiency. Surprisingly, the expression of its target FOXM1 did not correlate with the cytotoxic effects induced by thiostrepton in AML cells but was down-regulated in M2-macrophages upon treatment. Thiostrepton-treated healthy CD34+ cells remained largely unaffected in terms of cell viability and differentiation capacity, suggesting that thiostrepton may have a favorable therapeutic window.
In conclusion, our screening setup allows for the identification of compounds that can target both leukemic blasts as well as their tumor supportive microenvironment, and thiostrepton, KPT-9274 and metformin emerged as potentially promising candidates. We hope that (combinatorial) targeted drug treatments that also take the tumor microenvironment into account can help to improve AML treatment and prevent relapse.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal